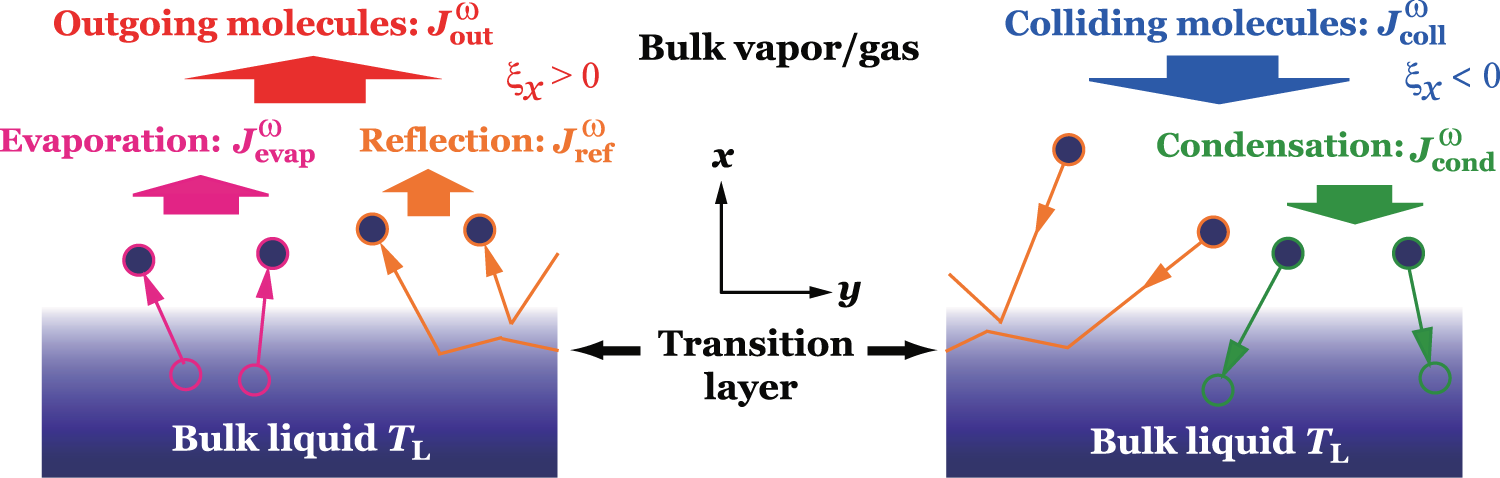
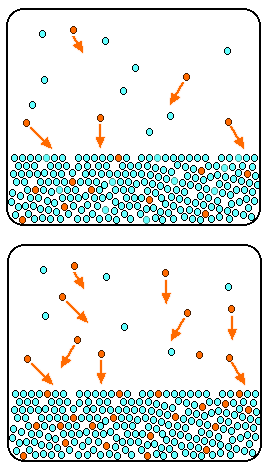
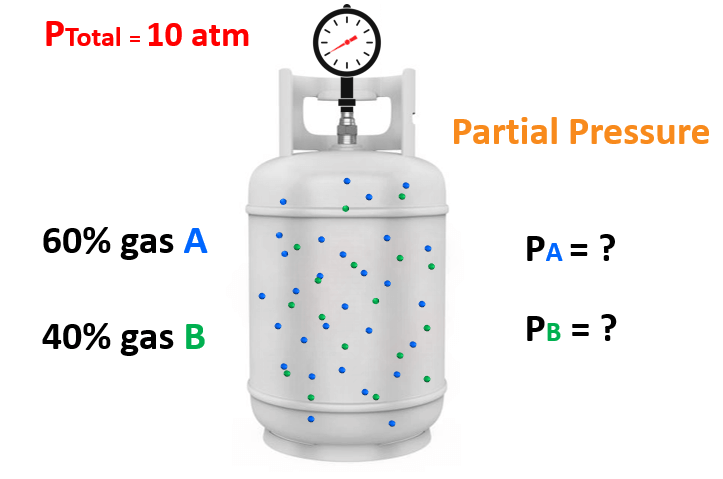
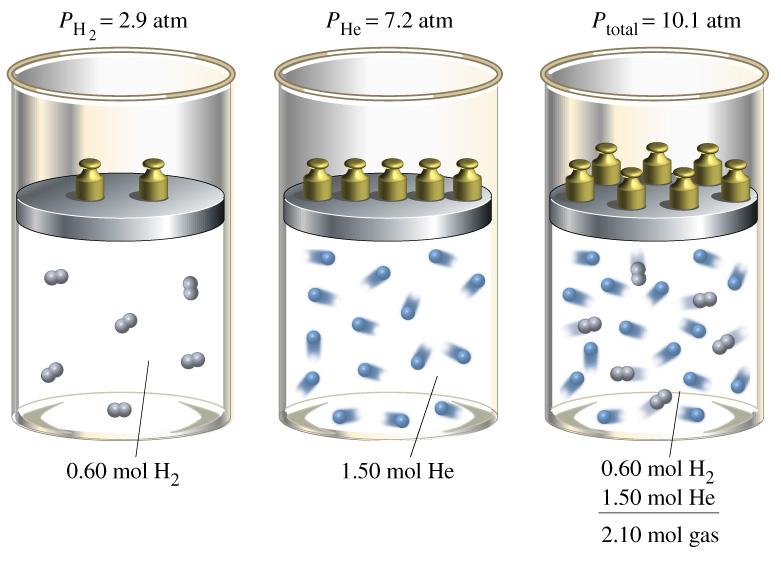
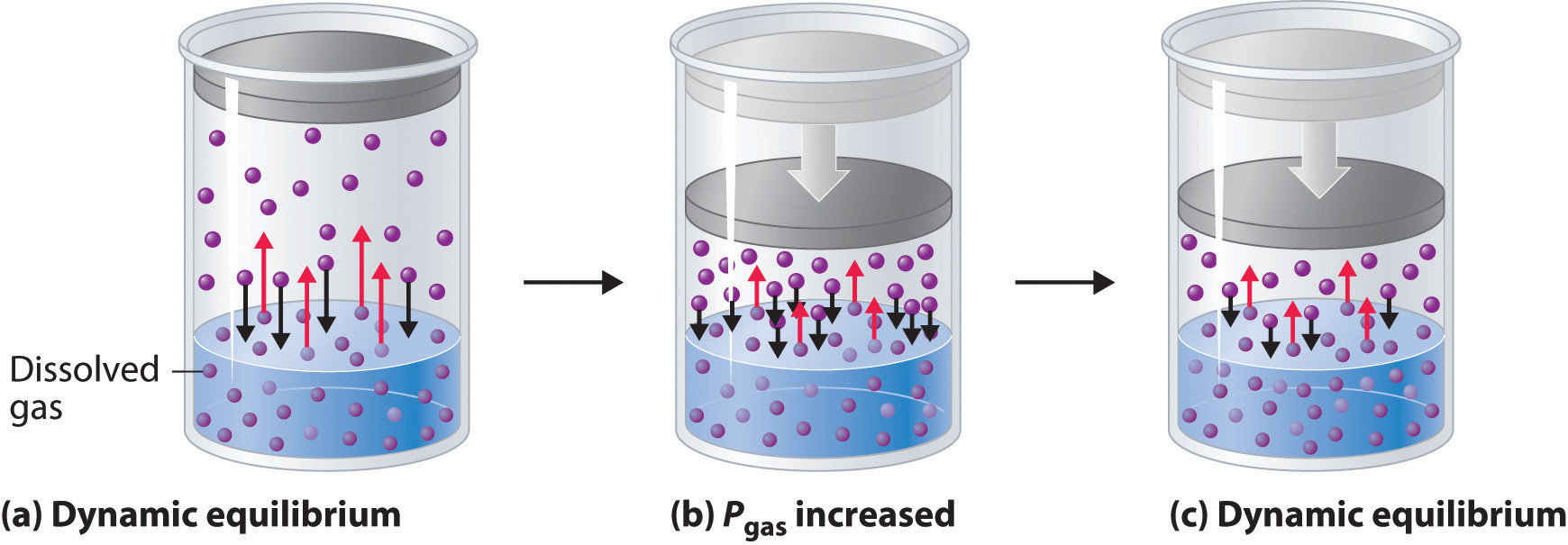
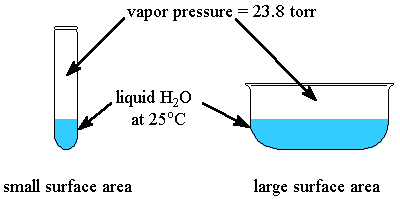
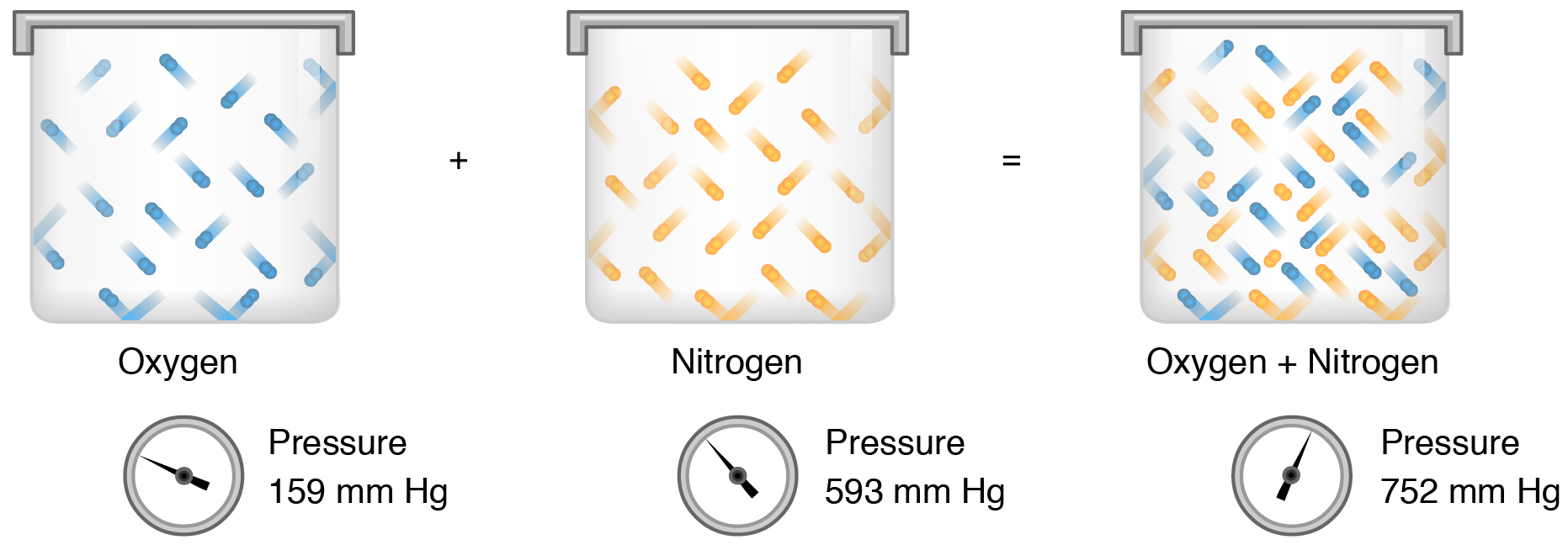
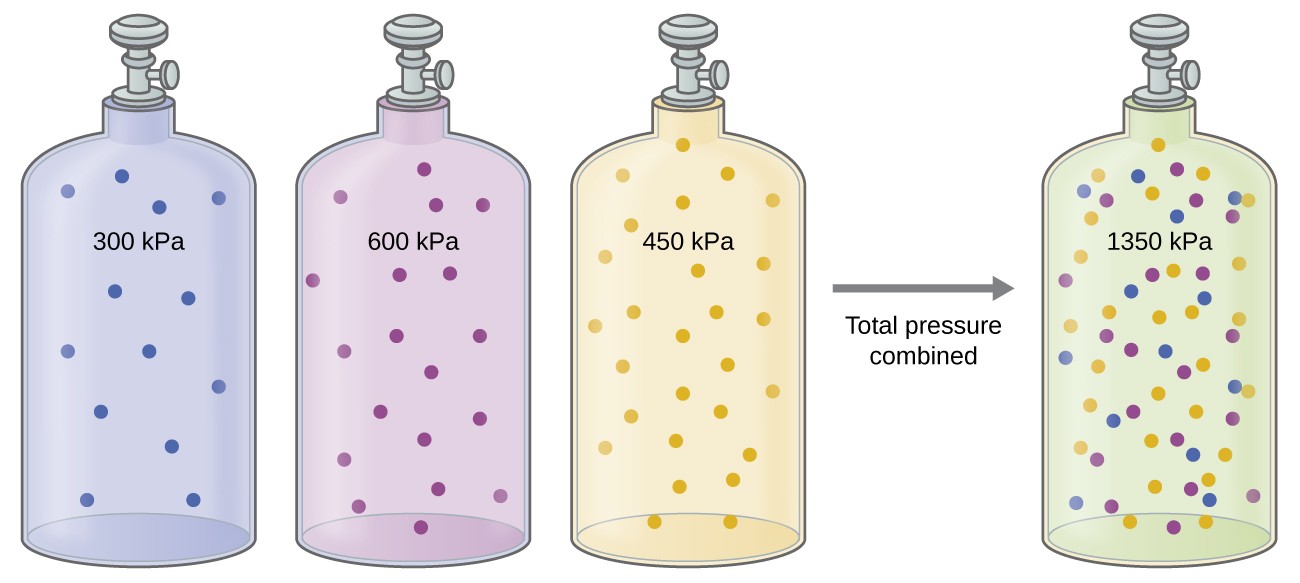
The relation between solubility of a gas in liquid at constant temperature and external pressure is stated by which law?
A tank of gas has partial pressures of nitrogen and oxygen equal to 1.61 xx 10^4 "kPa" and 4.34 xx 10^5 "kPa", respectively. What is the total pressure of the tank? | Socratic

CSAN Review of Principles. Daltons law and Partial Pressure In a mixture of gases, each gas has a partial pressure which is the pressure which the gas. - ppt download
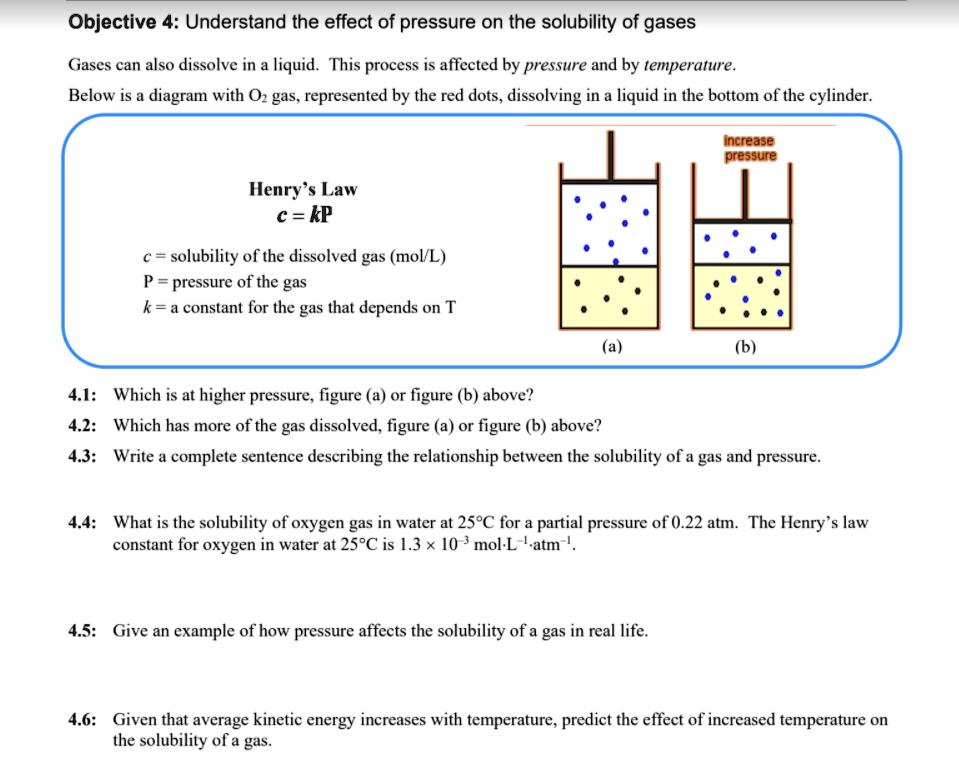
SOLVED: Objective 4: Understand the effect of pressure on the solubility of gases Gases can also dissolve in a liquid. This process is affected by pressure and by temperature: Below is a