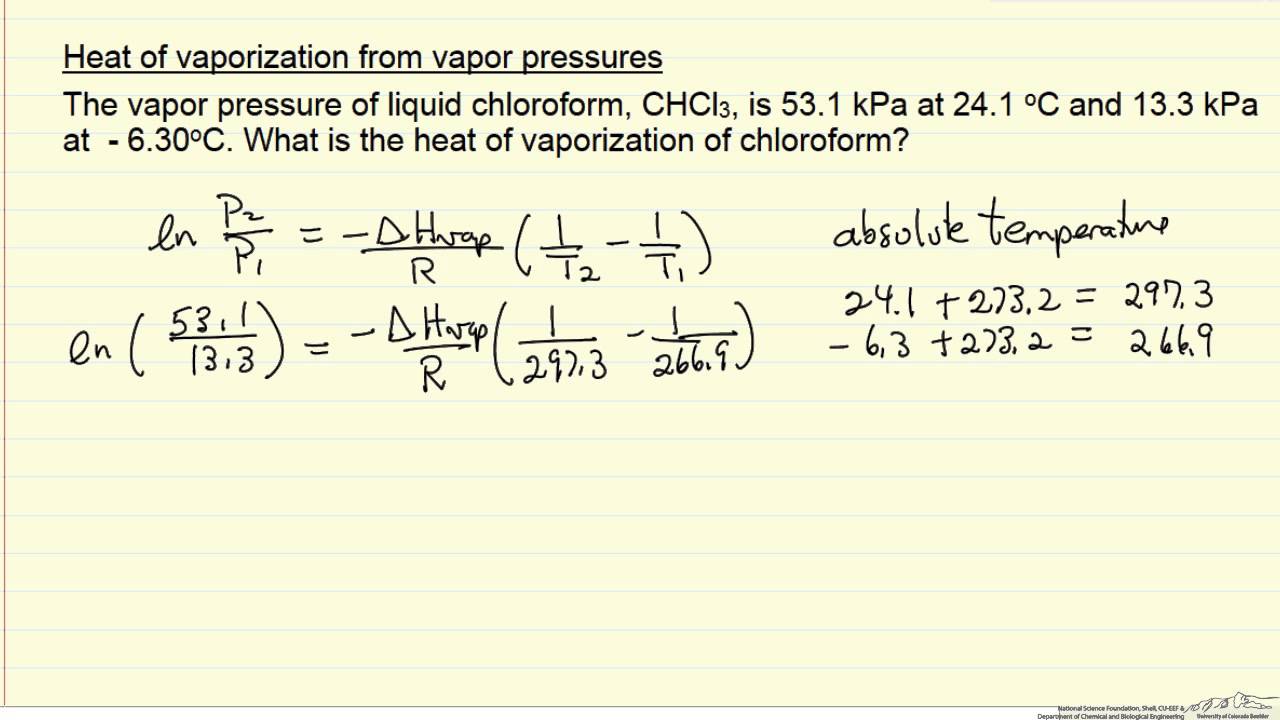
The enthalpy of vaporization of water at 100^o C is 40.63 KJ mol^-1 . The value Δ E for this process would be:

SOLVED: 7_ The enthalpy of vaporization of water at 100.0-C is 40.68 kJ mol. What is the entropy change in the surroundings when one mole of water vapor condenses at 100.0 %C

A theoretical analysis on enthalpy of vaporization: Temperature-dependence and singularity at the critical state - ScienceDirect

enthalpy - What is heat of vaporization? How can it be used at temperature as low as 25 °C? - Chemistry Stack Exchange
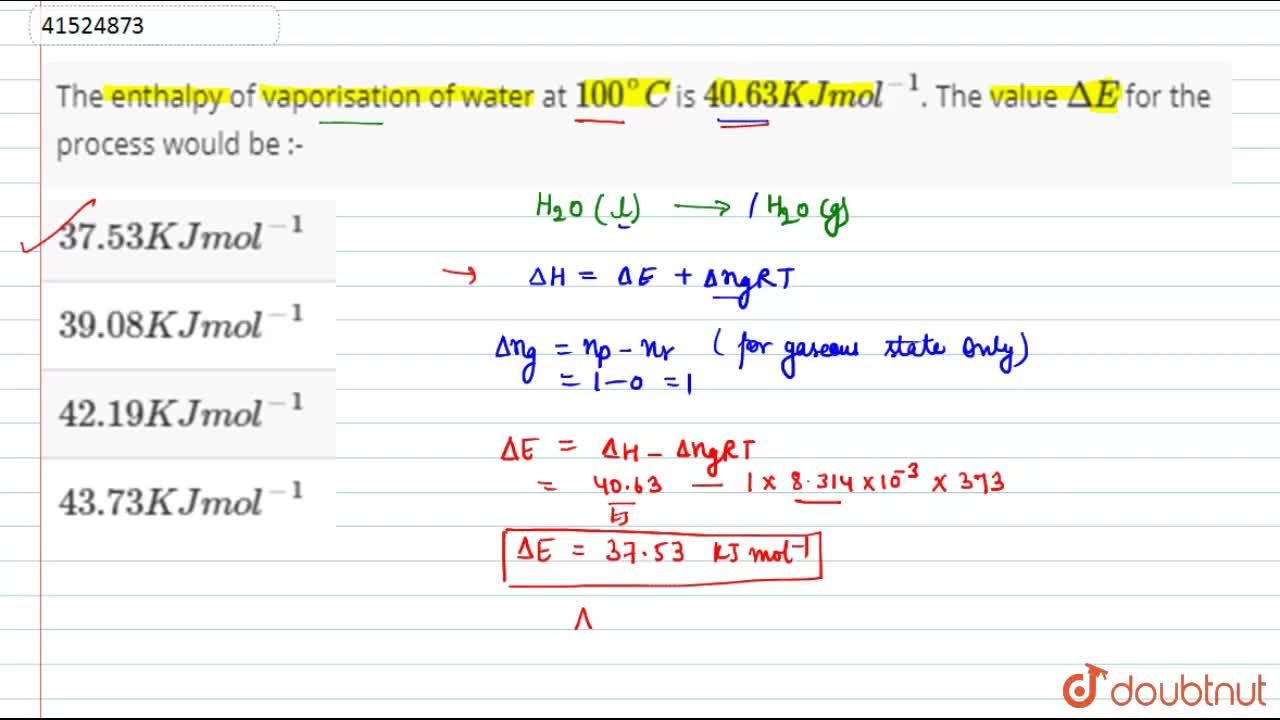
The enthalpy of vaporisation of water at 100^(@)C is 40.63 KJ mol^(-1). The value Delta E for the process would be :-
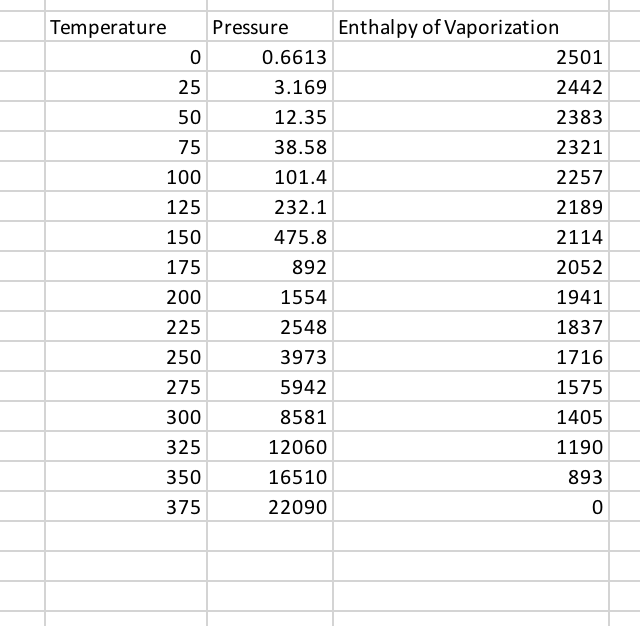
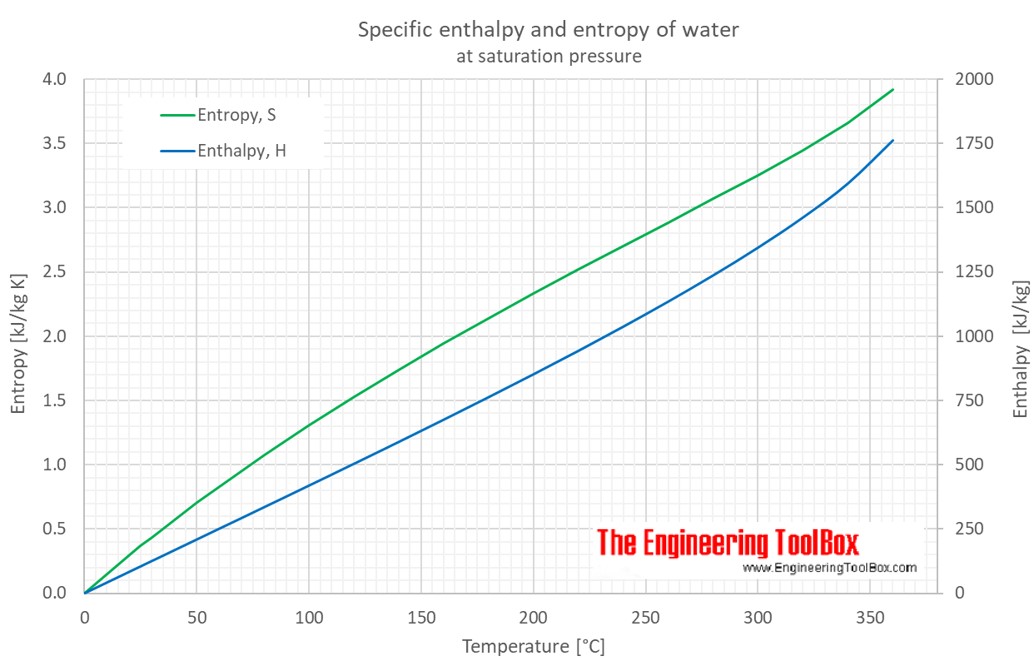
Molar enthalpy of vaporization of water from triple to critical points. | Download Scientific Diagram